
So with that p I of export eight This two points on nine, which is a PK of the boxer group, and 3.86 which is the PKK of the side chain average Those two hour gets US 2. And if we look more at it, we see that the peak a of the her boxer group and the surgeon or the most similar. Um, we see that it does have a night I will side chain.
Calculate pi of amino acid chain plus#
So we got 2.21 plus nine point 15 over to which gets us five point 68 on finally with us or Tate. So because of that, the P I is going to be the average of the two of the amino group and the box over. So again, Syrian, it doesn't have an ionized herbal side chain, no ionized a bull beside chain. So with that, um, solving for Syrian should and export, it should be pretty easy. And with that, we find that the amino group and the side chain our most similar and that we get 10.76 as a p I for burgeoning. So because of that, we need to take a look and see which to PK a values are the most similar. And I just gave it away, Right? So Argentine does have on ionizing side chain. So if we go to the table in the book, um, we'll see that Arjun has three values, right? One for the Kobach Sel group, one for the amino group, and then one for the ionizing side chain. Okay, Now, let's go ahead and take a look at urging. P I is equal to 2.2 plus 8.84 over to not equals 5.43 Now that is your P I for s perigee.

So because of that, if we scroll up here since this doesn't have an ionized excited change, the P I is the average of the two groups. Huh? And ionizing such a change with China is going to abbreviate as s e. So when we look at the table on, we see that experience isn't it is not a nine I thing or brother does not have on ionizing site chain. Okay, so let's take a look at espirit gene. And to see this in actual is go ahead and solve this question. So basically, what that means is that the two groups that have that are similar in PK value is what the p I is going to be the average of so the third thing we need to consider is if no then the p I with the ice electric point IHS the average of the to crooks.
Calculate pi of amino acid chain code#
APKHAY ) One Letter Code Three Letter Code Theoretical Isoelectric Point: - The isoelectric point is defined as the pH at which a particular amino acid sequence (peptide chain) bears no net electrical charge.

AlaGlyTyrGlu) or one letter code of amino acid sequence (e.g. So if yes, then the he I or ice electric point is the average oh, the who could and values uh, the similar ionizing groups. Isoelectric point Calculator Enter the three letter code (e.g. So if this is step one, this is stuff, too. Okay, so there's one most important thing we need to consider. So first we need to consider The first thing is, does the, um, you know, acid have ionized role? Uh, in I visible side chain. Before we go to that, there's who criteria, um, that we need to consider before solving this. Okay, now with that were asked to find the ice Electra points of experience, Argentine, Syrian and birth date. So there's no not charged do to a certain pH. So you look drink point and what's so special about this point? Well, at this point, it is when there IHS no net charge in the molecule on the specifically happens when, um, the pH is a certain point. So with that, what does it mean by the P I and what does that stand for? So the P I is also known as the ice.

It's important to know what on the P I IHS.
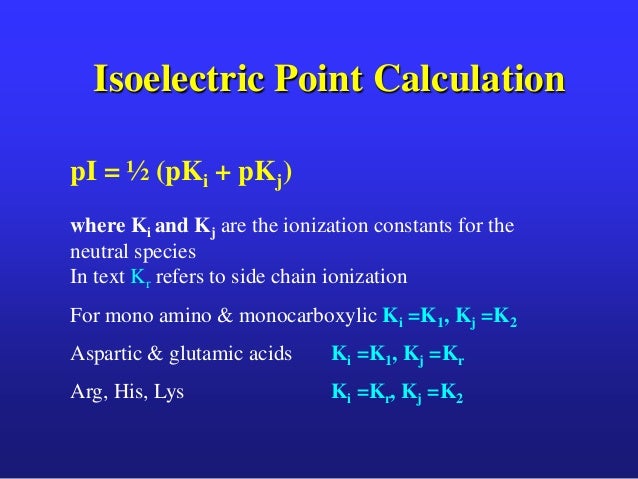
For acidic amino acids, the isoelectric point will be at lower pH as the acidic side chain will introduce an extra negative charge and for basic amino acids, the isoelectric point will be at higher pH as the basic side chain will introduce an extra positive charge.For this question. For neutral amino acids, the side chains are neutral and the isoelectric point is given simply by the average of the pK a values of carboxylic acid and amine. Note: The isoelectric point is given by the average of the pK a values that involve the zwitterions, not just by the pK a values that describe the carboxylic acid group and the amine group. Since the isoelectric point is given by the average of the pK a values that involve the zwitterion, so we can write the formula for lysine as:


 0 kommentar(er)
0 kommentar(er)
